Nation
Gujarat halts production at two pharma firms over sub par cough syrup batches
After the deaths of 14 children in MP, Gujarat acts against two companies after their syrups were found 'Not of Standard Quality'
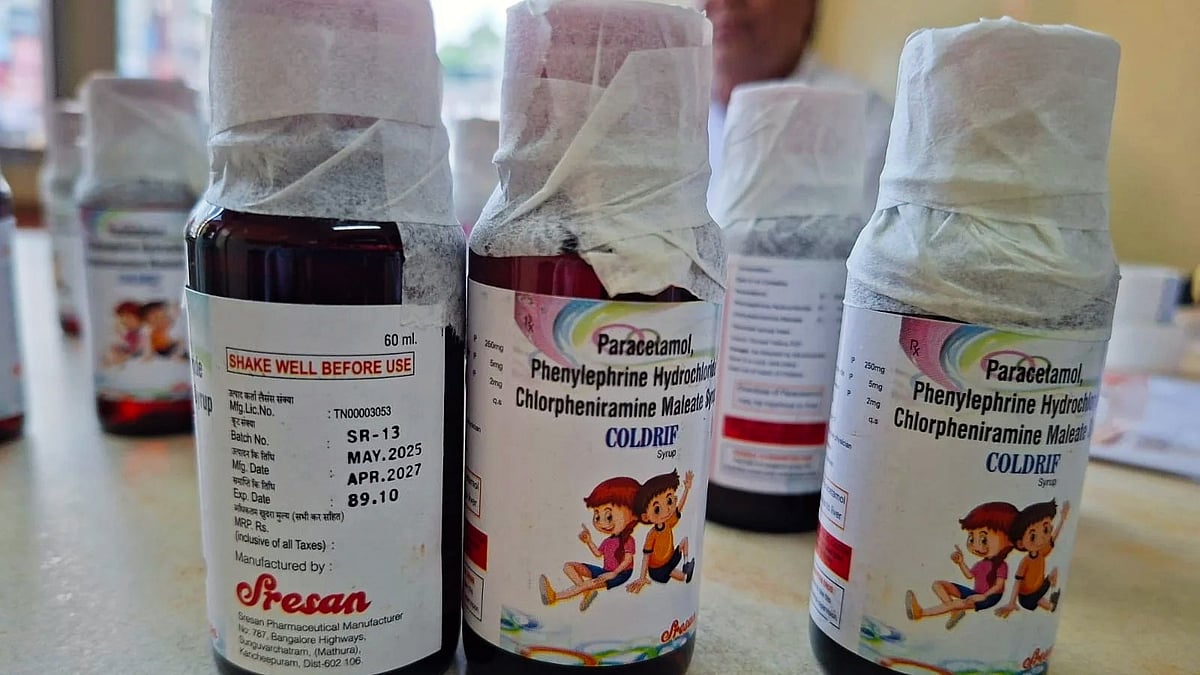
The Gujarat government has ordered an immediate suspension of production at two pharmaceutical companies after their cough syrups and other medicines were found to be NSQ (Not of Standard Quality) by health and regulatory authorities.
This action comes in the wake of the deaths of 14 children in Madhya Pradesh’s Chhindwara district, who are suspected to have died from renal failure linked to the consumption of a 'toxic' cough syrup, Coldrif.
Health minister Rushikesh Patel confirmed the developments on Tuesday, stating that Gujarat-based Shape Pharma Pvt Ltd, based in Surendranagar, and Rednex Pharmaceuticals Pvt Ltd from Ahmedabad, had been ordered to cease all operations.
The decision followed an investigation into the firms’ products and came shortly after the state government initiated a probe to determine whether syrups sold in Gujarat contained harmful ingredients, amid reports of child fatalities in Madhya Pradesh and Rajasthan allegedly due to contaminated cough syrups.
"Recently, some cough syrups meant for children and sold over the counter in Rajasthan, Uttar Pradesh, and Madhya Pradesh, have been declared 'NSQ' by authorities in those states. Some of these NSQ syrups were produced in Gujarat by two firms — Shape Pharma and Rednex Pharmaceuticals," Patel explained to reporters.
A joint team from the Gujarat Food and Drugs Control Administration (FDCA) and the Central Drugs Standard Control Organization (CDSCO) conducted an intensive inspection of the manufacturing facilities of both companies on 3 and 5 October. Based on the findings of the investigation, the authorities ordered the suspension of all medicine production at both facilities.
Published: undefined
Despite the suspension, Patel reassured the public that no NSQ products were found on-site during the inspection. He added, "We have also ordered both the firms to immediately recall the NSQ syrups from the market. We have asked chemists across the state not to sell these products and to return them."
Fourteen samples of other cough syrups produced by the two companies were collected during the inspection and sent for analysis at a government laboratory.
Gujarat is home to 624 licensed oral liquid medicine manufacturing companies, which distribute their products both within the state and outside. In light of these developments, the FDCA has issued strict instructions to all assistant commissioners to ensure that medicines manufactured and distributed in Gujarat meet safety and quality standards.
Patel stated, "The assistant commissioners have been instructed to conduct a strict inspection of all oral liquid or cough syrup manufacturing companies in their area. During this inspection, water quality, source of raw materials, production processes, quality control systems, and other critical factors will be thoroughly examined."
Officials have also been instructed to take at least five samples of different brands of oral liquid or cough syrup and test them promptly at the government laboratory in Vadodara.
Published: undefined
Follow us on: Facebook, Twitter, Google News, Instagram
Join our official telegram channel (@nationalherald) and stay updated with the latest headlines
Published: undefined