Cough syrups to be tested for export following several deaths overseas
After several cough syrups made in India were linked to several deaths of children in The Gambia and Uzbekistan, the union government has now made it compulsory to test them before exports

After several cough syrups made in India were linked to several deaths of children in The Gambia and Uzbekistan, the union government has now made it compulsory to test them before exports.
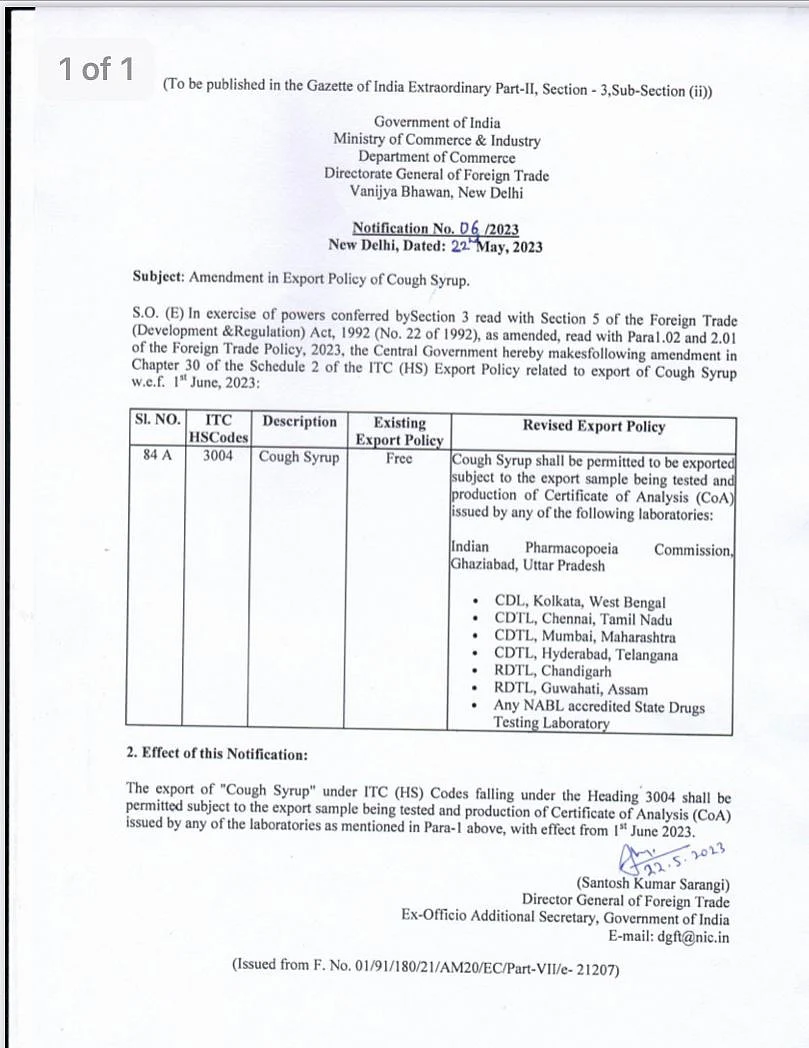
The revised export policy notification states that cough syrups can be exported only after the export sample is tested and the production certificate of analysis (CoA) is issued by one of the labs prescribed by the government.
In the notification issued by the Directorate General of Foreign Trade (DGFT), dated 22 May 2023, the government says that the certification should be obtained from Indian Pharmacopoeia Commission, Regional Drug Testing Lab (RDTL - Chandigarh), Central Drugs Lab (CDL - Kolkata), Central Drug Testing Lab (CDTL - Chennai, Hyderabad, Mumbai), RDTL (Guwahati) and the NABL (National Accreditation Board for Testing and Calibration Laboratories) accredited drug testing labs of State governments.
The direction has come after quality concerns were raised globally for cough syrups exported by Indian firms.
“The finished goods are to be tested at laboratories before being permitted for export and the required steps are being taken to ensure implementation of the tests and their requirement,” said an official of the health ministry.
This comes after the World Health Organisation, in October 2022, drew a link between the deaths of at least 66 children in Gambia and the four India-made cough syrups. WHO Director-General Tedros Adhanom Ghebreyesus, had then said, “The four medicines are cough and cold syrups produced by Maiden Pharmaceuticals Limited in India. WHO is conducting further investigation with the company and regulatory authorities in India," he said, adding that the loss of young lives due to the products is "beyond heart-breaking for their families".
The four products were Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup. The manufacturer of these products is Maiden Pharmaceuticals Limited, Haryana, India, and to date, the stated manufacturer had not provided guarantees to WHO on the safety and quality of these products, WHO said.
The WHO Medical Product Alert referred to four substandard products, identified in The Gambia and reported to WHO in September 2022. Laboratory analysis of samples of each of the four products confirmed that they contain unacceptable amounts of diethylene glycol and ethylene glycol as contaminants.
Then in December 2022, WHO again issued a warning against the use of two other cough syrups which were made in India and were linked to deaths of at least 20 children in Uzbekistan. WHO specified that products named Ambrenol and DOK-1 Max, which were made by Marion Biotech were of substandard quality. Then the union health ministry had suspended the production at the company.
Here too, WHO had said that the laboratory analysis of these products found unacceptable amounts of diethylene glycol and /or ethylene glycol as contaminants”.
Outlining the risks associated with the products, WHO had then said diethylene glycol and ethylene glycol are toxic to humans when consumed and can prove fatal. “Toxic effects can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state and acute kidney injury which may lead to death,” it said.
In February, Chennai-based Global Pharma Healthcare had recalled 50,000 tubes of eye drops in the US market due to bacterial contamination as it was linked to vision loss in the US. The US Food and Drug Administration (USFDA) said the “FDA analysis found unopened tubes to be contaminated with bacteria”.
India is the largest provider of generic drugs globally, supplying over 50 per cent of global demand for various vaccines, about 40 per cent of generic demand in the US and about 25 per cent of all medicine in the UK. Globally, India ranks third in terms of pharmaceutical production by volume and 14th by value.
With PTI inputs
Follow us on: Facebook, Twitter, Google News, Instagram
Join our official telegram channel (@nationalherald) and stay updated with the latest headlines
